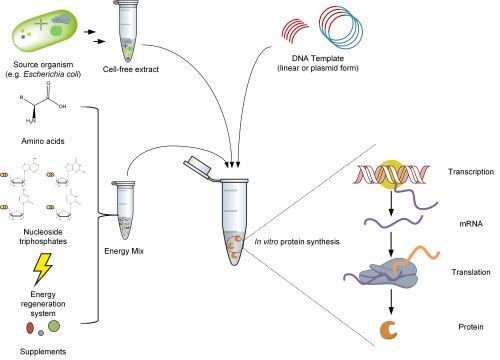
Cell-free protein synthesis
For the development of bioprocesses, the screening of suitable biocatalysts is essential and a complex task [1]. Therefore, the aim of my study is to develop a high-throughput screening system for biocatalysts. The speed-limiting step in biocatalyst library generation is usually heterologous expression and protein purification. Therefore, the use of cell-free protein synthesis (CFPS) in an automated setup can circumvent this bottleneck and increase the efficiency of bioprocess development.
Cell-free systems have been used for protein synthesis for decades and offer a wide range of applications and several advantages over heterologous expression. Proteins can be produced from DNA templates within a few hours with concentrations in the milligram per milliliter range [2]. The CFPS mix consists of three parts:
- A cell-free extract containing all the necessary components for the coupled transcription and translation machinery, such as ribosomes, aminoacyl-tRNA synthetases, and translation factors for initiation, elongation, and product release.
- The DNA template containing the gene of interest.
- An energy mix with the building blocks for mRNA and protein synthesis, as well as additional cofactors and supplements.
Rational improvement of the complementary components leads to systems that enable novel CFPS applications beyond pure research interests. Therefore, the integration of CFPS into platforms for high-throughput enzyme screening and analysis can serve as a powerful and versatile tool for the rapid discovery of new candidates or improved biocatalyst variants.
[1] Lütz, S., Giver, L., & Lalonde, J. (2008). Engineered enzymes for chemical production. Biotechnology and Bioengineering, 101(4), 647-653. doi [2] Caschera, F., & Noireaux, V. (2014). Synthesis of 2.3 mg/ml of protein with an all Escherichia coli cell-free transcription-translation system. Biochimie, 99(1), 162-168. doi